FDA approves 'game-changing' prostate cancer tool
TAMPA, Fla. - Prostate cancer is the most common cancer among men with one in eight developing it in their lifetime. Now, a new diagnostic tool approved by the FDA on Thursday is changing the way physicians treat prostate cancer patients by more accurately pinpointing where the cancer spread.
Moffitt Cancer Center was one of the nationwide locations that took part in phase III clinical trials for the product.
"I think it’s going to make a big difference in ways we can’t even foresee yet," said Dr. Kenneth Gage, an imaging specialist at Moffitt Cancer Center and local principal investigator.
When a man’s prostate cancer comes back after initial treatment, the next step is to figure out where it is in the body.
Before now, spotting those spreading cancer cells in a patient's body was difficult because traditional scans do not detect enough. If doctors do not know where the cancer is, they do not know how to effectively manage it.
"All of these different treatments have risks and benefits and complications that people have to deal with," Gage said. "And so we don’t want to treat people inappropriately, you want to give the right treatment, to the right spot at the right time."
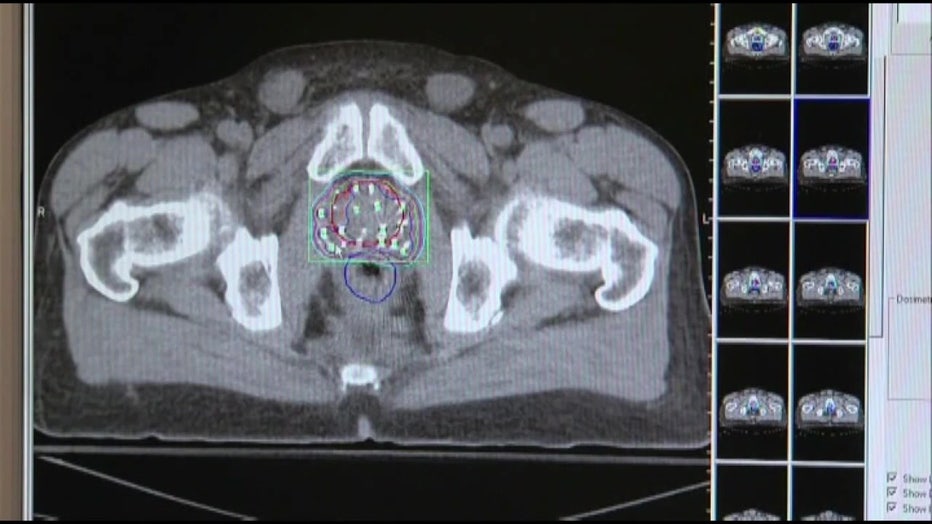
The new imaging agent, PYLARIFY, is the most sensitive way to find prostate cancer in the body and is a huge advancement. The cancer cells that spread have a unique marker called Prostate-Specific Membrane Antigen, or PSMA. The injection binds to the PSMA, lighting up on a PET scan, revealing exactly where the cancer cells are in the body. Much sooner and more precise than other scans.
"Detecting it earlier allows us to catch it before it has the opportunity to grow further, grow faster and mutate more," Gage explained.
READ Study finds high levels of cancer-causing chemical in several sunscreen brands
Having more complete information about where the cancer is located, and how bad it is, means a man’s doctor can design a treatment plan specifically for him and save lives.
"I think it’s going to allow us to give much more tailored, much more thoughtful, much more accurate treatment than we’ve been able to do in the past," Gage said.
"It’s the best thing that we have right now."
It is expected to have a significant impact on men battling the disease.
This is the first tracer for recurrent prostate cancer commercially available nationwide.
Using PYLARIFY with a scan is not meant to replace PSA testing, which is a common prostate cancer screening tool.
CONNECT WITH FOX 13:


